Chemicals
Re: Chemicals
I found elements like fluorine and chlorine interesting in chemistry class. Needing that one electron to complete a shell. Also those with one in a shell that it is wanting to bond like sodium.
Re: Chemicals
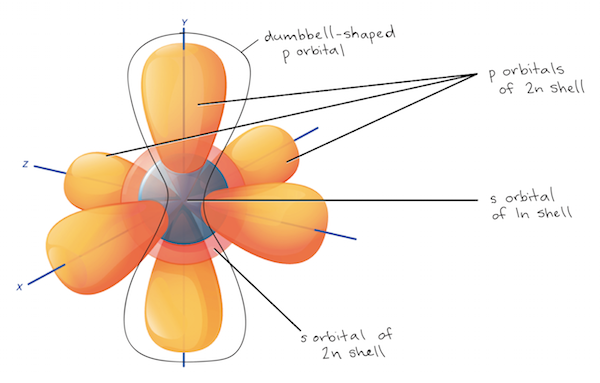
The first electron shell, 1n, corresponds to a single 1s1s1s1, s orbital. The 1s1s1s1, s orbital is the closest orbital to the nucleus, and it fills with electrons first, before any other orbital. Hydrogen has just one electron, so it has a single spot in the 1s1s1s1, s orbital occupied. This can be written out in a shorthand form called an electron configuration as 1s11s^ 11s11, s, start superscript, 1, end superscript, where the superscripted 1 refers to the one electron in the 1s1s1s1, s orbital. Helium has two electrons, so it can completely fill the 1s1s1s1, s orbital with its two electrons. This is written out as 1s21s^ 21s21, s, squared, referring to the two electrons of helium in the 1s1s1s1, s orbital. On the periodic table, hydrogen and helium are the only two elements in the first row, or period, which reflects that they only have electrons in their first shell. Hydrogen and helium are the only two elements that have electrons exclusively in the 1s1s1s1, s orbital in their neutral, non-charged, state.
The second electron shell, 2n, contains another spherical ssss orbital plus three dumbbell-shaped pppp orbitals, each of which can hold two electrons. After the 1s1s1s1, s orbital is filled, the second electron shell begins to fill, with electrons going first into the 2s2s2s2, s orbital and then into the three pppp orbitals. Elements in the second row of the periodic table place their electrons in the 2n shell as well as the 1n shell. For instance, lithium (Li\text{Li}Listart text, L, i, end text) has three electrons: two fill the 1s1s1s1, s orbital, and the third is placed in the 2s2s2s2, s orbital, giving an electron configuration of 1s21s^ 21s21, s, squared 2s12s^ 12s12, s, start superscript, 1, end superscript. Neon (Ne\text{Ne}Nestart text, N, e, end text), on the other hand, has a total of ten electrons: two are in its innermost 1s1s1s1, s orbital and eight fill the second shell—two each in the 2s2s2s2, s and three pppp orbitals, 1s21s^ 21s21, s, squared 2s22s^ 22s22, s, squared 2p62p^62p62, p, start superscript, 6, end superscript. Because its 2n shell is filled, it is energetically stable as a single atom and will rarely form chemical bonds with other atoms.
The third electron shell, 3n, also contains an ssss orbital and three pppp orbitals, and the third-row elements of the periodic table place their electrons in these orbitals, much as second-row elements do for the 2n shell. The 3n shell also contains a dddd orbital, but this orbital is considerably higher in energy than the 3s3s3s3, s and 3p3p3p3, p orbitals and does not begin to fill until the fourth row of the periodic table. This is why third-row elements, such as argon, can be stable with just eight valence electrons: their ssss and pppp subshells are filled, even though the entire 3n shell is not.
Link
Re: Chemicals
You applying the concepts of physics to auto-immune disease therapy or the grand simulation?
Re: Chemicals
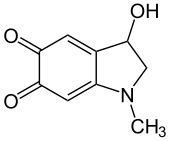
Adrenochrome
Adrenochrome is a chemical compound with the molecular formula C9H9NO3 produced by the oxidation of adrenaline (epinephrine). The derivative carbazochrome is a hemostatic medication. Despite a similarity in chemical names, it is unrelated to chrome or chromium.
Several small-scale studies (involving 15 or fewer test subjects) conducted in the 1950s and 1960s reported that adrenochrome triggered psychotic reactions such as thought disorder, derealization, and euphoria. Researchers Abram Hoffer and Humphry Osmond claimed that adrenochrome is a neurotoxic, psychotomimetic substance and may play a role in schizophrenia and other mental illnesses.
In what they called the "adrenochrome hypothesis", they speculated that megadoses of vitamin C and niacin could cure schizophrenia by reducing brain adrenochrome. While the treatment of schizophrenia with such potent anti-oxidants is highly contested in literature, and adrenochrome is not currently believed to have any psychedelic properties, a number of recently published papers consider Hoffer's paper a landmark contribution to the notion that impairment of what's now termed the anti-oxidant defense system (AODS) seems to play a role in schizophrenia
- Author Hunter S. Thompson mentioned adrenochrome in his book Fear and Loathing in Las Vegas. The adrenochrome scene also appears in the novel's film adaptation. In the DVD commentary, director Terry Gilliam admits that his and Thompson's portrayal is a fictional exaggeration. Gilliam insists that the drug is entirely fictional and seems unaware of the existence of a substance with even a similar name. Hunter S. Thompson also mentions adrenochrome in his book Fear and Loathing on the Campaign Trail '72. In the footnotes in chapter April, page 140 he says, "It was sometime after midnight in a ratty hotel room and my memory of the conversation is haze, due to massive ingestion of booze, fatback, and forty cc's of adrenochrome."
- In Anthony Burgess' 1962 novel A Clockwork Orange, "drencrom" (presumably the Nadsat term for adrenochrome) is listed as one of the potential drugs that can be added to Moloko Plus (milk laced with a drug of the consumer's choice) at the Korova Milk Bar.
- Adrenochrome is also featured in a variety of conspiracy theories, such as QAnon and Pizzagate.[12]
https://www.google.com/search?q=Adrenoc ... 20&bih=976

Re: Chemicals
How to Draw Lewis Dot Structures
Re: Chemicals
Have you seen the adrenochrome threads and conspiracy?